Momelotinib [N-(cyanomethyl)-4-[2-[[4-(4-morpholinyl)phenyl]amino]-4-pyrimidinyl]-benzamide]
is a small-molecule, ATP-competitive and highly selective inhibitor for Janus
Kinase 1 (JAK1, IC50 =
11 nM) and Janus Kinase 2 (JAK2, IC50 = 18
nM). Momelotinib inhibited JAK1 and JAK2 equipotently, but had
an IC50 that was approximately ninefold higher for
the closely related JAK3 (IC50 = 155 nM) kinase as compared
with JAK2.
 |
| Momelotinib: 2D and 3D Structure |
In a
‘single-point’ screening assay that assesses the degree of enzyme inhibition at
a specified Momelotinib concentration (100 nM or 1 uM), only eight
kinases (JAK1, JAK2, CDK2/A, ROCK2, MAPK8, TBK1, PRKD1 and PRKCN) showed less
than 50% at 100 nM [1].
Summary
Common name: CYT387; CYT 387; CYT-387; CYT 11387;
CYT-11387; CYT11387
Trademarks:
Molecular Formula: C23H22N6O2
CAS Registry Number: 1056634-68-4
CAS Name: N-(Cyanomethyl)-4-[2-(4-morpholinoanilino)pyrimidin-4-yl]benzamide
Molecular Weight: 414.47
SMILES:O=C(NCC#N)C1=CC=C(C2=NC(NC3=CC=C(N4CCOCC4)C=C3)=NC=C2)C=C1
InChI Key: ZVHNDZWQTBEVRY-UHFFFAOYSA-N
InChI: InChI=1S/C23H22N6O2/c24-10-12-25-22(30)18-3-1-17(2-4-18)21-9-11-26-23(28-21)27-19-5-7-20(8-6-19)29-13-15-31-16-14-29/h1-9,11H,12-16H2,(H,25,30)(H,26,27,28)
Activity: Treatment of myelofibrosis; Anti-neoplastics
Drug; Anti-inflammatory Agents
Status: Phase I/II
Originator: Gilead Sciences
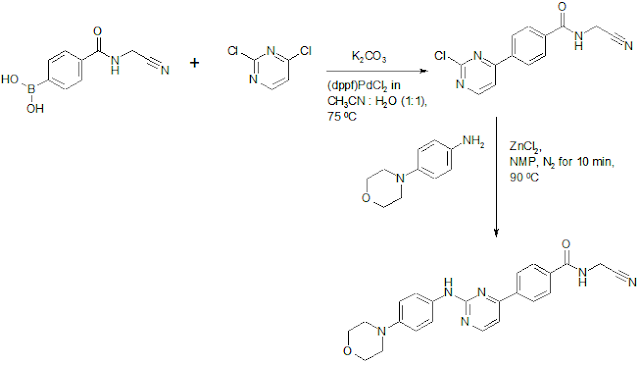
Momelotinib Synthesis
US8486941B2: This is first disclosed synthesis for Momelotinib. It doesn't appear to be industrially optimized.
Identifications:
References:
1. Pardanani, A.; et. al. CYT387, a selective JAK1/JAK2 inhibitor: in vitro assessment of kinase selectivity and preclinical studies using cell lines and primary cells from polycythemia vera patients. Leukemia 2009, 23(8), 1441-1445.
2. Brown, B. H.; et. al. N-(cyanomethyl)-4-(2-(4-morpholinophenylamino)pyrimidin-4-yl)benzamide hydrochloride salts. WO2015191846A1
 |
| 1H NMR (Estimated) for Momelotinib |
 |
| 13C NMR (Estimated) for Momelotinib |
Experimental: 13C
NMR (75.5 MHz, d6-DMSO) δ 166.04, 162.34, 160.26, 159.14,
146.14, 139.87, 134.44, 132.73, 127.80, 126.84, 120.29, 117.49, 115.50, 107.51,
66.06, 49.16, 27.68.
References:
1. Pardanani, A.; et. al. CYT387, a selective JAK1/JAK2 inhibitor: in vitro assessment of kinase selectivity and preclinical studies using cell lines and primary cells from polycythemia vera patients. Leukemia 2009, 23(8), 1441-1445.
2. Brown, B. H.; et. al. N-(cyanomethyl)-4-(2-(4-morpholinophenylamino)pyrimidin-4-yl)benzamide hydrochloride salts. WO2015191846A1
3. Burns, C. J.; et. al. Phenyl amino pyrimidine compounds and uses thereof. US8486941B2