Seratrodast
[7-Phenyl-7-(2,4,5-trimethyl-3,6-dioxocyclohexa-1,4-dien-1-yl)heptanoic
acid] is a thromboxane A2 (TXA2) receptor {TP
receptor} antagonist marketed as an antiasthmatic drug with inhibitory effects
on immediate asthmatic reaction in asthma and airway hypersensitivity. It also
has antagonistic effects on contractile prostanoids, including PGH2,
PGF2α, PGD2, and 11β- PGD2. Thus this drug is
expected to have a wide range of antagonistic effects on bronchoconstriction
mediators [1, 2].
 |
| 2D and 3D Structure for Seratrodast |
Chemically,
it is a quinone derivative with a long alkyl chain ending in a carboxylic acid,
making it poorly soluble in water. It is a chiral molecule, though marketed as
a racemate, studies report the best activities in the following order of
descent: R–enantiomer, racemate and finally S–enantiomer. It is a novel design,
orally active, potent and long acting TP/PGH2 receptor antagonist.
Seratrodast
does not affect thrombus formation, time to occlusion and bleeding time. Seratrodast
has no effect on prothrombin time and activated partial thromboplastin time,
thus ruling out any action on blood coagulation cascade [1].
It was
the first TP receptor antagonist that was developed as an anti-asthmatic drug
and received marketing approval in Japan in 1997. Seratrodast is currently
marketed in Japan, China and India (approved in December 2012) as an add-on
controller therapy in the management of asthma.
Pharmacological Importance of Thromboxane and
Prostaglandin
Thromboxane
(TX) A2 and prostaglandin (PG) I2 (prostacyclin) are two
of the metabolites of arachidonic acid produced through the cyclooxygenase
pathway. These two compounds produce potent but opposite effects on smooth
muscle and on platelets. Thromboxane A2 (TXA2) is a type
of thromboxane that is produced by activated platelets and has prothrombotic
properties: it stimulates activation of new platelets as well as increases
platelet aggregation. Prostacyclin (also called prostaglandin I2 or
PGI2) is a prostaglandin member of the eicosanoid family of lipid
molecules. It inhibits platelet activation and is also an effective
vasodilator [3].
The
production of thromboxane and/or prostacyclin has been altered in patients with
various diseases, such as unstable angina, glomerular disease secondary to
systemic lupus erythematosus, systemic sclerosis complicated by Raynaud’s
phenomenon, septic shock, and asthma. Increased levels of TXA2
metabolites have been found in the urine and in bronchoalveolar lavage fluid of
atopic asthmatics after allergen challenge. These data suggest that treatment
with an agent that inhibits TX synthesis or blocks TX receptors could have
beneficial effects in these pathologies.
Seratrodast
blocks the broncho-constrictor effects of prostaglandins in the body.
Seratrodast also decreases the inflammation by antagonising the thromboxane A2
receptor. Addition of a TXA2 receptor antagonist to conventional
antiasthma medications may be considered in the management of patients with
mild to moderate asthma having increased airway secretions.
Mechanism of Action
Seratrodast,
a quinone derivative, is a novel, potent, orally active, and long acting TXA2/PGH2
receptor antagonist. In addition, Seratrodast has PGF2α, PGD2,
and 9α, 11β -PGF2 antagonistic effects [1, 4].
The
specificity of Seratrodast at antagonizing the TXA2 receptor was
tested in vitro, ex vivo, and in vivo.
Because of the short half-life of TXA2 (about 30 s), more stable
analogs such as U-46619 and U-44069 were used to study the antagonistic effect
of Seratrodast on TXA2 receptors. Similar to TXA2, such
analogs exert a potent platelet-aggregating and smooth muscle contracting
effect. Several studies in animals were conducted to investigate the
pharmacological effects of Seratrodast in these areas and its antagonistic
effect on the TXA2 receptor.
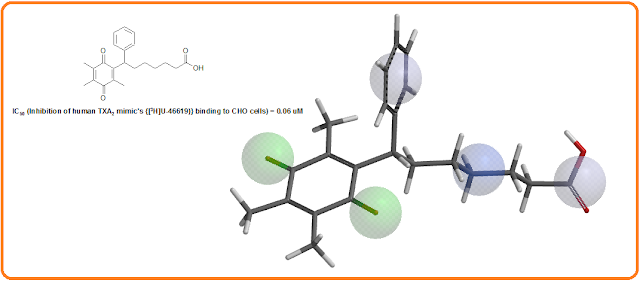
The
specific binding of Seratrodast to platelets was tested in guinea pigs and
humans. Seratrodast inhibited the specific binding of [3H]U-46619 to
washed guinea pig platelets with an IC50 value of 0.0082 uM. This
inhibition was more effective than the inhibition produced by unlabeled U-46619
(IC50 = 0.037 uM). Moreover, the antagonistic effect of Seratrodast
was tested on the human TXA2 receptor genetically introduced into
Chinese hamster ovary (CHO) cells. Seratrodast competitively inhibited the
specific binding of TXA2 mimic ( [3H]U-46619) to the
ovary cells with an IC50 of 0.06 uM, indicating that the drug is an
antagonist of human TXA2 receptor [1, 4].
Seratrodast
had neither an anti- Leukotriene
D4 (LTD), nor an anti- platelet-activating
factor (PAF) activity at 100 uM (guinea pig lung membranes) or 10 uM (rabbit
platelets), respectively.
The
specific antagonism of Seratrodast against the different enzymes involved in
arachidonic acid metabolism was also tested. Seratrodast inhibited the
production of 5-hydroxyeicosatetraenoic acid from arachidonic acid
(5-lipoxygenase antagonism) by rat basophilic leukemia cells with an IC50
value of 0.21 uM. A very weak inhibitory effect of Seratrodast was reported on
thromboxane synthetase (horse platelet, 13% inhibition at 10 uM) and
cyclooxygenase (bovine vesticular gland, 20% inhibition at 100 uM).
Dosages and Approvals
Seratrodast
(Tradename: Bronica) was approved in
Japan in 1997 for treatment of bronchial asthma at once daily doses of 80 mg to
be administered after dinner. Subsequently
China and India (in 2012) have approved the drug an add-on controller therapy
in the management of asthma. Seratrodast has been well tolerated following
repeated once daily oral doses of up to a maximum of 320 mg. In elderly
patients it is recommended that the treatment should be started with a lower
dose of 40 mg/day. Phase trials were carried out in the west but it has not
been approved by EU or US till date.
Seratrodast was discovered, developed and launched by Takeda Pharmaceuticals, Japan.
Seratrodast was discovered, developed and launched by Takeda Pharmaceuticals, Japan.
Reported Activities for Seratrodast
Platelet Aggregation: In the in vitro studies, Seratrodast inhibited
the aggregation of guinea pig platelets induced by a prostaglandin endoperoxide
(PGH,) analog, U-44069, arachidonic acid, or collagen in a
concentration-dependent manner with IC50 values of 0.31, 0.49 and
0.23 uM respectively. Seratrodast did not affect the platelet activating factor
(PAF) or adenosine-5’-diphosphate (ADP)-induced aggregation. Seratrodast at a
concentration of nearly 900 uM did not induce shape change or platelet
aggregation, indicating that it has no agonistic action.
In ex vivo experiments, Seratrodast by oral
administration (0.1-1.0 mg/kg) inhibited in a dose-dependent manner the
platelet aggregation induced by U-44069 in guinea pigs and the rabbit platelet
aggregation induced by arachidonic acid [1].
Antithrombotic effect: The activity of Seratrodast was also tested in
male New Zealand rabbits were common carotid artery thrombosis induced by
endothelial damage was significantly inhibited by oral administration of
seratrodast. Seratrodast had little effect (16%) on increased plasma
concentration of 11-dehydro TXB2 a stable metabolite of TXA2.
The in vivo antithrombotic effect of Seratrodast
was significantly correlated (p < 0.01) with its ex vivo inhibitory effects on arachidonic acid-induced platelet
aggregation in rabbits. This correlation suggests that Seratrodast produce its
antithrombotic effect by inhibiting the binding of TXA2 to the
receptor [1].
The
prothrombin time and activated partial thromboplastin time were not affected by
high doses of Seratrodast (10 mg/kg), ruling out any direct inhibition of blood
coagulation cascade.
Vascular Smooth Muscle: Seratrodast
competitively inhibited the contractile response to U-46619 in guinea pig lung
parenchymal strips and dog saphenous vein strips with pA2 mean values
of 8.29 ± 0.09 and 6.79 ± 0.05, respectively. Similarly, Seratrodast
competitively inhibited the contraction of rabbit aorta and pig coronary
arteries induced by U-44069 with pA2 values of 8.3 and 9.0,
respectively. Seratrodast also inhibited the contraction of rabbit aorta
induced by PGF2α (pA2 = 7.8) and the contraction of pig
coronary arteries induced by PGF2α, PGD2, and 9α, 11β -PGF2
a metabolite of PGD2 with pA2 values of 7.8, 8.6,
and 7.8, respectively [1].
Airway bronchoconstriction and asthma: Seratrodast
competitively inhibited the contractile response to U-46619, PGD2,
and 9α, 11β -PGF2 or PGF2α with pA2 values of
7.69, 7.20, 7.79, and 5.71, respectively. The contractile responses of guinea
pig tracheal strips to leukotriene D4 (LTD4) or platelet
activating factor (PAF) were not inhibited by Seratrodast. Seratrodast given
orally inhibited the bronchoconstriction in guinea pigs induced by U-46619 (10 ug/kg,
i.v.), LTD4 (10 ug/kg i.v.), or PAF (1 ug/kg i.v.). Seratrodast did not inhibit
bronchoconstriction induced by histamine (10 ug/kg i.v.) in guinea pigs [1].
The
effects of racemic Seratrodast and its enantiomers were compared in vitro and in vivo. In vitro, racemic
Seratrodast and the R (+)-enantiomer were more potent than the S(-)-enantiomer.
Also, oral administration of racemic Seratrodast and the R(+) enantiomer showed
very potent inhibitory effects on U-46619-induced bronchoconstriction in guinea
pigs in vivo (minimum effective dose
(MED] = 0.3 mg/kg), whereas the S(-)-enantiomer was much less active (MED = 20
mg/kg).
Activities for racemic Seratrodast,
R-enantiomer and S-enantiomer
pA2
(Inhibiting U-44069-induced contractions of rabbit aorta) = 8.28, 8.60 and 6.64
pA2
(Inhibiting U-46619-induced contraction of the guinea pig lung) = 8.29 ± 0.09,
IC50
(Inhibition of U-44069-induced aggregation of guinea pig platelets) = 0.35, 0.12 and 13 uM
IC50
(Inhibiting specific binding of [3H]U-46619 to guinea pig platelets)
= 0.0074, 0.0021 and 0.21 uM
Summary
Common name: AA-2414; AA2414; AA
2414; ABT-001; A-73001; Abbott-73001; Seratrodast
Trademarks: Bronica
Molecular Formula: C22H26O4
CAS Registry Number: 112665-43-7; 103187-09-3 (R-isomer); 103196-89-0 (S-isomer)
CAS Name: 7-phenyl-7-(2,4,5-trimethyl-3,6-dioxocyclohexa-1,4-dien-1-yl)heptanoic
acid
Molecular Weight: 354.44
SMILES:O=C(O)CCCCCC(C1=CC=CC=C1)C2=C(C)C(C(C)=C(C)C2=O)=O
InChI Key: ZBVKEHDGYSLCCC-UHFFFAOYSA-N
InChI: InChI=1S/C22H26O4/c1-14-15(2)22(26)20(16(3)21(14)25)18(17-10-6-4-7-11-17)12-8-5-9-13-19(23)24/h4,6-7,10-11,18H,5,8-9,12-13H2,1-3H3,(H,23,24)
Mechanism of Action: Thromboxane A2
(TXA2) receptor antagonist; Prostaglandin H2 (PGH2)
receptor antagonist
Activity: Management of asthma; Anti-asthmatic
drug; Anti-inflammatory; Non-antihistaminic
Status: Launched 1997 (Japan)
Chemical Class: Small molecules; Benzene containing; Carboxylic acid containing; Long chain alkyls; Quinone derivative
Originator: Takeda Pharmaceuticals/Abbott Pharma


