Common name: Clopidogrel bisulfate, Clopidogrel hydrogensulfate, DV-7314, SR-25990C
Trademarks: Plavix
Molecular Formula: C16H16ClNO2S. H2O4S
CAS Registry Number: 120202-66-6, 113665-84-2 (free base), 94188-84-8 (racemic;free base)
Molecular Weight: 419.9042
CAS Name: (+)-(S)-2-(2-Chlorophenyl)-2-(4,5,6,7-tetrahydrothieno[3,2-c]pyridin-5-yl)acetic acid methyl ester hydrogensulfate
Activity: Antiplatelet Therapy, Atherosclerosis Therapy, CARDIOVASCULAR DRUGS, Cerebrovascular Diseases, Treatment of, Coagulation Disorders Therapy, HEMATOLOGIC DRUGS, NEUROLOGIC DRUGS, Stroke, Treatment of, Treatment of Disorders of the Coronary Arteries and Atherosclerosis, P2Y12 (P2T) AntagonistsIntermediates:dl-(2-Chlorophenyl)glycine (141196-64-7)
dl-2-Chloromandelic Acid (10421-85-9)
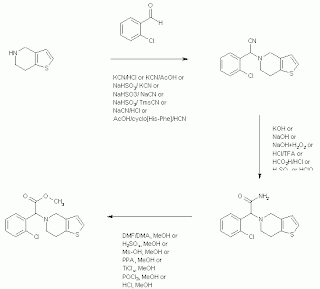
Clopidogrel synthesis: Drug of Future, 1993, 18(2), 107
Clopidogrel synthesis: WO2002059128A2
(S)-Clopidogrel synthesis: US48747265
Clopidogrel synthesis: Org Process Res Dev 2007, 11(3), 487-489
Clopidogrel synthesis: J Am Chem Soc, 2013, 135(43), 16074–16077 (one step, starting material commercially available)
- Lohray, V.B.; et. al. Process for preparing clopidogrel. WO2002059128A2
- Descamps, M.; et. al. Process for the preparation of an N-phenylacetic derivative of tetrahydrothieno(3,2-c)pyridine and its intermediate of synthesis. EP0446569
- Savi, P.; et. al. Clopidogrel Hydrogensulfate. Drugs Fut 1993, 18(2), 107
- Wang, L.; et. al. Synthetic Improvements in the Preparation of Clopidogrel. Org Process Res Dev 2007, 11(3), 487-489.
- MacMillan, D. W. C.; et. al. A Simple Catalytic Mechanism for the Direct Coupling of a-Carbonyls with Functionalized Amines: A One-Step Synthesis of Plavix. J Am Chem Soc 2013, 135(43), 16074-16077.
For more drugs and their synthesis: Synayurajan database