Carfilzomib [(S)-4-Methyl-N-((S)-1-(((S)-4-methyl-1-((R)-2-methyloxiran-2-yl)-1-oxopentan-2-yl)amino)-1-oxo-3-phenylpropan-2-yl)-2-((S)-2-(2-morpholinoacetamido)-4-phenylbutanamido)pentanamide] is a selective, irreversible tetrapeptide epoxyketone inhibitor of the 20S
proteasome, which has been granted accelerated approval in the US for the
treatment of patients with multiple myeloma (MM). Those administered should have
received at least two prior therapies, including Bortezomib and an
immunomodulatory agent (Thalidomide or Lenalidomide), and have demonstrated
disease progression on or within 60 days of completion of the last therapy [1].
Carfilzomib
displays high selectivity for and irreversibly inhibits the chymotrypsin-like
catalytic activity of the 20S proteasome core particle, which results in cell
growth arrest and apoptosis.
 |
| Carfilzomib: 2D and 3D Structure |
Various
clinical trials proved that Carfilzomib had an acceptable tolerability profile
in patients with relapsed, or relapsed and refractory, multiple myeloma. There
was a low incidence of grade 3 or higher peripheral neuropathy.
Bortezomib, a peptide boronate, was the first proteasome inhibitor to be approved and demonstrated very good antimyeloma activity. Bortezomib, was initially approved by the United States Food and Drug Administration (FDA) in 2003 for the treatment of relapsed and refractory disease in patients who have received at least 2 prior therapies. It subsequently received FDA approval as a firstline therapy for patients with relapsed or refractory MM. Bortezomib inhibits proteasomes by binding reversibly to the chymotrypsin- like catalytic site in the core 20S particle. However, resistance to Bortezomib is emerging, and adverse effects, such as painful peripheral neuropathy and thrombocytopenia have restricted Bortezomib to a biweekly day 1/day 4 dosing schedule that allows full recovery of proteasome activity between doses. This has limited Bortezomib’s use in some patients, suggesting a need for new-generation inhibitors.
Therefore, clinical evaluation of additional proteasome inhibitor classes is warranted. Two irreversible proteasome inhibitors are currently under development: (a) salinosporamide A (NPI-0052), a natural product related to lactacytsin and (b) Carfilzomib (also as PR-171), a modified peptide related to the natural product epoxomicin [2].
The Proteasome as a Therapeutic Target for Multiple
Myeloma (MM)
Eukaryotic
cells contain substantial amounts of a large proteolytic complex called the
proteasome that is responsible for the ubiquitin-dependent turnover of cellular
proteins. Proteasome substrates include misfolded or misassembled proteins as
well as short-lived components of signaling cascades that regulate cell
proliferation and survival pathways. The proteasome is also responsible for
protein degradation in highly regulated processes including cell-cycle
progression, inflammatory responses, such as NF-kB activation, and antigen
processing. Inhibition of the proteasome results in the accumulation of these
substrate proteins and leads to cell death.
The proteasome exists in two isoforms: the constitutive proteasome, which exists in most cells; and the inducible proteasome (i.e. immunoproteasome), which is mainly expressed in lymphoid cells.
The catalytic core of
the proteasome includes three proteolytic activities that are commonly
described by their substrate selectivities: chymotrypsin-like, trypsin-like,
and caspase-like. Each proteasome active site uses the side chain hydroxyl
group of an NH2-terminal threonine as the catalytic nucleophile, a
mechanism that distinguishes the proteasome from other cellular proteases [2].The proteasome exists in two isoforms: the constitutive proteasome, which exists in most cells; and the inducible proteasome (i.e. immunoproteasome), which is mainly expressed in lymphoid cells.
Bortezomib, a peptide boronate, was the first proteasome inhibitor to be approved and demonstrated very good antimyeloma activity. Bortezomib, was initially approved by the United States Food and Drug Administration (FDA) in 2003 for the treatment of relapsed and refractory disease in patients who have received at least 2 prior therapies. It subsequently received FDA approval as a firstline therapy for patients with relapsed or refractory MM. Bortezomib inhibits proteasomes by binding reversibly to the chymotrypsin- like catalytic site in the core 20S particle. However, resistance to Bortezomib is emerging, and adverse effects, such as painful peripheral neuropathy and thrombocytopenia have restricted Bortezomib to a biweekly day 1/day 4 dosing schedule that allows full recovery of proteasome activity between doses. This has limited Bortezomib’s use in some patients, suggesting a need for new-generation inhibitors.
Therefore, clinical evaluation of additional proteasome inhibitor classes is warranted. Two irreversible proteasome inhibitors are currently under development: (a) salinosporamide A (NPI-0052), a natural product related to lactacytsin and (b) Carfilzomib (also as PR-171), a modified peptide related to the natural product epoxomicin [2].
From Epoxomicin to YU-101; and then finally
to Carfilzomib
The α', β'-epoxyketone containing natural product
epoxomicin was isolated from an Actinomycetes
strain based on its in vivo antitumor
activity against murine B16 melanoma tumors [3]. Despite this potent activity,
the mechanism of epoxomicin’s biological action has remained unknown. Later, researchers
identified the proteasome as the intracellular protein target of this potent
antitumor agent. Using a synthetic biotinylated affinity derivative, it was
shown that epoxomicin covalently binds the LMP7, X, Z, and MECL1 catalytic β subunits of the
proteasome and selectively inhibits the three major proteasome proteolytic
activities at different rates [4].
Epoxomicin
and its analogues are comprised of two key elements: a peptide portion that
selectively binds in the substrate binding pocket(s) of the proteasome with
high affinity and an epoxyketone pharmacophore that stereospecifically
interacts with the catalytic threonine residue to irreversibly inhibit enzyme
activity. X-ray crystallography has shown that epoxomicin forms a dual covalent
morpholino adduct with the proteasome that requires the close juxtaposition of
both the side chain hydroxyl and a-amino groups of the active site threonine
residue [5]. This unique mechanism imparts a high degree of specificity to the
proteasome relative to the active sites of other protease classes.
Medicinal chemistry efforts focused on increasing the potency and chymotrypsin-like selectivity of epoxomicin resulted in the identification of YU-101 [6], a synthetic tetrapeptide epoxyketone analogue. The peptide epoxyketone YU-101 is a potent and selective inhibitor of the chymotrypsin-like activity of the 20S proteasome (kobs/[I] (M–1s–1 = 5-12 nM). However, the low aqueous solubility of this compound (less than 1 µg/mL) limits its utility in vivo. Carfilzomib (also as PR-171), is an analogue of YU-101 that exhibits improved aqueous solubility (greater than 1000-fold) due to the introduction of an NH2-terminal morpholino moiety.
Medicinal chemistry efforts focused on increasing the potency and chymotrypsin-like selectivity of epoxomicin resulted in the identification of YU-101 [6], a synthetic tetrapeptide epoxyketone analogue. The peptide epoxyketone YU-101 is a potent and selective inhibitor of the chymotrypsin-like activity of the 20S proteasome (kobs/[I] (M–1s–1 = 5-12 nM). However, the low aqueous solubility of this compound (less than 1 µg/mL) limits its utility in vivo. Carfilzomib (also as PR-171), is an analogue of YU-101 that exhibits improved aqueous solubility (greater than 1000-fold) due to the introduction of an NH2-terminal morpholino moiety.
 |
| Structure and Activity of Epoxomicin and YU-101 |
Dosages and Approval:
Carfilzomib
(Tradename: Kyprolis) is approved as
an injection, for intravenous use in the
treatment of patients with multiple myeloma (MM) who have received at least two
prior therapies including bortezomib and an immunomodulatory agent and have
demonstrated disease progression on or within 60 days of completion of the last
therapy. The U.S. Food and Drug Administration (FDA) approved it on 20 July
2012.
Carfilzomib
is administered intravenously over 2 to 10 minutes, on two consecutive days, each
week for three weeks (Days 1, 2, 8, 9, 15, and 16), followed by a 12-day rest
period (Days 17 to 28). Each 28-day period is considered one treatment cycle.
In
Cycle 1, Carfilzomib is administered at a dose of 20 mg/m2. If
tolerated in Cycle 1, the dose should be escalated to 27 mg/m2
beginning in Cycle 2 and continued at 27 mg/m2 in subsequent cycles.
Treatment may be continued until disease progression or until unacceptable
toxicity occurs. The dose is calculated using the patient’s actual body surface
area at baseline. Patients with a body surface area greater than 2.2 m2 should
receive a dose based upon a body surface area of 2.2 m2. Dose
adjustments do not need to be made for weight changes of less than or equal to
20%.
After
reconstitution with sterile water, Carfilzomib solution (2 mg/mL) should be
administered intravenously over 2-10 minutes, and not as a bolus. In order to
reduce the incidence and severity of infusion reactions, patients should be premedicated
with oral or intravenous Dexamethasone 4 mg before each dose of Carfilzomib in
cycle one and before each Carfilzomib dose in the first cycle of dose
escalation to 27 mg/m2. In subsequent cycles, Dexamethasone
premedication should be reintroduced if infusion reaction symptoms occur.
The
researchers from Bristol-Myers Squibb Research Institute in Tokyo who
discovered epoxomicin knew it had potent in vivo antitumor activity, but they
had no idea how it worked. It is researchers at Yale University, namely Craig
M. Crews who found epoxomicin as a fascinating compound-a microbial natural product
which was a tetrapeptide with an unusual epoxy ketone group at one end. They
found that epoxomicin was binding to the proteasome-the biochemical machinery
that slices and dices damaged or unwanted proteins. Then the idea clicked, to
use proteasome inhibition to develop a drug and epoxomicin analog YU-101 was
developed. Along with Caltech professor Raymond J. Deshaies, Crews started
South San Francisco-based Proteolix, where they added a morpholine ring to the YU-101
to boost its solubility, thereby creating Carfilzomib.
Proteolix advanced Carfilzomib
to multiple Phase 1 and 2 clinical trials, including a pivotal Phase 2 clinical
trial designed to seek accelerated approval. In 2009 Onyx Pharmaceuticals, acquired
Proteolix, and continued Phase 3 trials. In January 2011, the FDA granted Carfilzomib
fast-track status, allowing Onyx to initiate a rolling submission of its new
drug application for Carfilzomib [7].
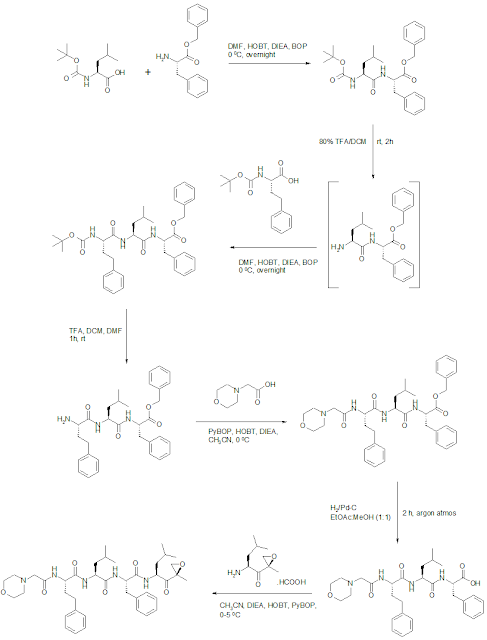
Carfilzomib Synthesis
WO2009045497A1: Allylic ketones are stereoselectively converted to the desired keto epoxides. The patent reports the scheme specifically for Carfilzomib.
Intermediate Keto-epoxyamine: Reaction of N-Boc leucine with isobutyl chloroformate followed by N,O-dimethylhydroxylamine provided Weinreb amide in 94% yield. Grignard addition of isopropenylmagnesium bromide provided enone in 81% yield. Epoxidation of enone with calcium hypochlorite provided a mixture of epoxides giving 41% yield of the desired isomer (presumably isolated by chromatography), and subsequent treatment with TFA liberated the amine, providing the TFA salt of ketoepoxy amine in 92% yield.
WO2005105827A2: The patent reports an alternate route to Carfilzomib synthesis.
Alternate method to synthesize Keto-epoxyamine intermediate: This is first reported procedure for keto-epoxyamine intermediate. Isomer b was used further to synthesize epoxomicin.
Bioorg Med Chem Lett 1999, 9, 2283-2288: Synthesis
of the keto-epoxy fragment was initiated with the addition of propen-2-yl
lithium to the Boc-leucine Weinreb amide which resulted in formation of the alpha,
beta-unsaturated ketone. Subsequent
epoxidation with alkaline hydrogen peroxide furnished a mixture of the epoxides
a and epoxide b (1:1.7), which were readily separated by column chromatography.
Both epoxides a and epoxide b were brought forward to give epoxomicin and its
epoxide epimer. It was concluded that epoxide b gave a final product identical
to epoxomicin.
Identifications:
 |
| 1H NMR (Estimated) for Carfilzomib |
References:
1. McCormack, P. L. Carfilzomib: In Relapsed, or Relapsed and Refractory, Multiple Myeloma. Drugs 2012, 72(15), 2023-2032. (FMO only)
2. Demo, S. D.; et. al. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res 2007, 67(13), 6383-6391. (free copy)
3. Hanada, M.; et. al. Epoxomicin, a new antitumor agent of microbial origin. J Antibiot (Tokyo) 1992, 45(11), 1746-1752. (free copy)
4. Meng, L.; et. al. Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity. Proc Natl Acad Sci U S A 1999, 96(18), 10403-10408. (free copy)
5. Groll, M.; et. al. Crystal structure of epoxomicin: 20S proteasome reveals a molecular basis of a',ß'-epoxyketone proteasome inhibitors. J Am Chem Soc 2000, 122(6), 1237-1238. (free copy)
6. Elofsson, M.; et. al. Towards subunit-specific proteasome inhibitors: synthesis and evaluation of peptide alpha',beta'-epoxyketones. Chem Biol 1999, 6(11), 811-822. (FMO only)
7. Halford, B. Carfilzomib: From Discovery To Drug. Chemical & Engineering News 2012, 90(35), 34-35. (free copy)