Common name: Darunavir, UIC-94017, TMC-114
Trademarks: Prezista (Tibotec)
Molecular Formula: C27H37N3O7S
CAS Registry Number: 206361-99-1
CAS Name: N-[3-[N-(4-Aminophenylsulfonyl)-N-isobutylamino]-1(S)-benzyl-2(R)-hydroxypropyl]carbamic acid (3R,3aS,6aR)-perhydrofuro[2,3-b]furan-3-yl ester
Molecular Weight: 547.6693
Activity: AIDS Medicines, Anti-HIV Agents, ANTIINFECTIVE THERAPY, HIV Protease Inhibitors
Darunavir synthesis: WO1999067254A2
Synthesis of Darunvair can be divided into two parts.
Part 1: Synthesis of bis-tetrahydrofuranyl alcohol (Many routes are available in the literature, will report as much as possible)
Scheme 1 (Tetrahedron Lett 1995, 36(4), 505-508)
Scheme 2 (Org Proc Res Dev 2007, 11, 972-980)
Scheme 3 (Paterno-Buchi photochemical route; WO2003024974A2)
Part 2: Epoxide synthesis
Scheme 1 (Synthesis 2001, 15, 2203-2229)
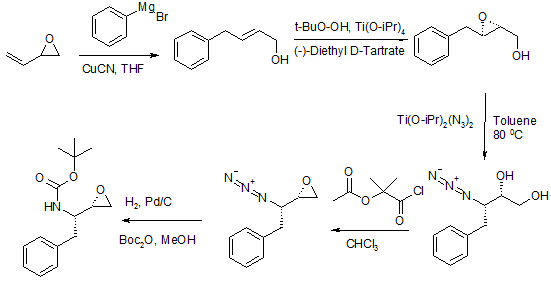
Scheme 2 (J Med Chem 1993, 36(16), 2300-2310)
Finally, Darunavir
A diastereomer of Darunavir is synthesized starting with D-phenyl alanine: Indian J Chem (Sec B) 2012, 51B, 849-854 (also see US20060148865A1, for the intermediate and also for a new potential HIV inhibitor)
References:
- Ghosh, A. K.; et. al. Potent HIV protease inhibitors incorporating high-affinity P2-ligands and (R)-(hydroxyethylamino)sulfonamide isostere. Bioorg Med Chem Lett 1998, 8, 687.
- Ghosh, A. K.; et. al. Multi-drug resistant retroviral protease inhibitors and associated methods. WO1999067254A2
- Ghosh, A. K.; et. al. Synthesis and optical resolution of high affinity P2-ligands for HIV-1 protease inhibitors Tetrahedron Lett 1995, 36(4), 505-508.
- Yu, R. H.; et. al. Research and Development of an Efficient Synthesis of Hexahydrofuro[2,3-b]furan-3-ol Moiety-A Key Component of the HIV Protease Inhibitor Candidates. Org Proc Res Dev 2007, 11, 972-980.
- Ghosh, A. K.; et. al. Syntheses of FDA Approved HIV Protease Inhibitors. Synthesis 2001, 15, 2203-2229.
- Davis, R. D.; et. al. Process for preparing protease inhibitor intermediates. WO2003024974A2
- Ghosh, A. K.; et. al. Potent HIV protease inhibitors: the development of tetrahydrofuranylglycines as novel P2-ligands and pyrazine amides as P3-ligands. J Med Chem 1993, 36(16), 2300-2310.